Merz Aesthetics announces UK approval of Bocouture® for upper facial lines
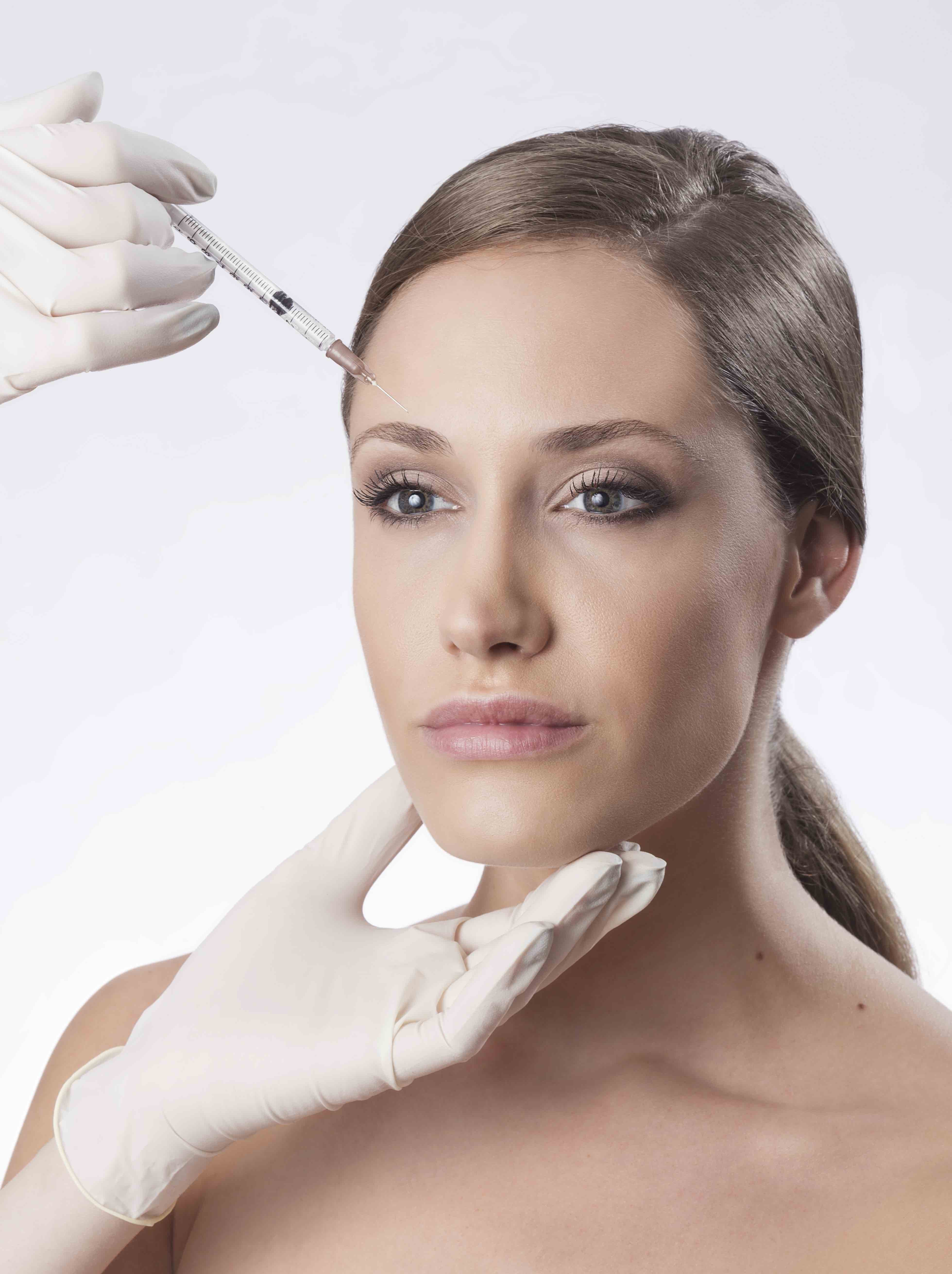 Bocouture® has been approved by the Medicines and Healthcare products Regulatory Agency (MHRA) for the treatment of upper facial lines, including horizontal frown lines, lateral periorbital lines and glabellar frown lines. Bocouture® is currently the only neurotoxin approved in the UK for this combined upper facial lines indication.
Bocouture® has been approved by the Medicines and Healthcare products Regulatory Agency (MHRA) for the treatment of upper facial lines, including horizontal frown lines, lateral periorbital lines and glabellar frown lines. Bocouture® is currently the only neurotoxin approved in the UK for this combined upper facial lines indication.
“Merz is a global leader in the aesthetics space and is proud to be able to provide patients and physicians in the UK with the first and only aesthetic neurotoxin approved for combination treatment of upper facial lines,” stated Stuart Rose, managing director of Merz Pharma UK.
Recent market research has indicated that the rejuvenation of upper facial lines is one of the most requested aesthetic procedures among both existing patients and those considering treatment. In clinical practice, many patients request combined treatment of upper facial lines in a single session to achieve optimal treatment outcomes.
“As the only neurotoxin treatment approved for the simultaneous treatment of upper facial lines, Bocouture® supports aesthetic practitioners in their day to day practice and enables them to provide their patients with well tolerated and effective treatments with confidence,” said Lucy Dowling, brand manager, Merz Aesthetics. “We are very proud of the fact that, while Bocouture® was not the first toxin to enter the UK aesthetics market, it is the first product to achieve this important milestone.”
The approval of this new indication is based on the results of a pivotal randomised, double-blind, placebo-controlled Phase III study with 156 patients from the UK, France and Germany receiving treatment of upper facial lines with Bocouture®. Clinical trial data demonstrate that Bocouture® has a favourable safety and efficacy profile in treating upper facial lines, both combined and separately, with treatment effects maintained for up to four months.

