New drug application Seysara is FDA approved
Published
23rd Dec 2017
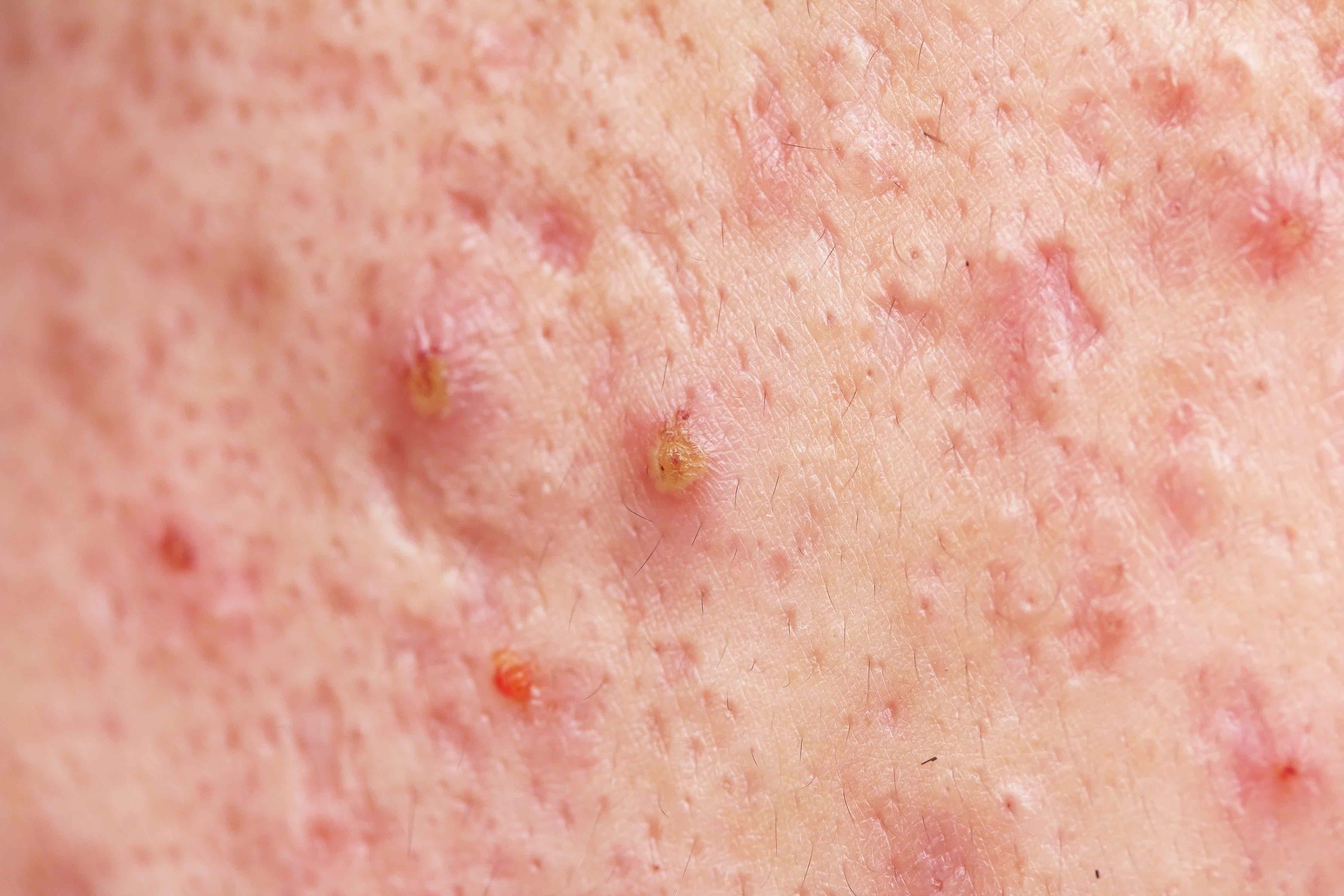
Leading global pharmaceutical company, Allergan, and biopharmaceutical company focused on the development and commercialisation of therapies based upon tetracycline chemistry, Paratek Pharmaceuticals Inc., announced that the FDA has accepted to review Seysara™ (Sarecycline) for the treatment of moderate to severe acne vulgaris in patients nine years of age and older.
Seysara™ (Sarecycline) is a once-daily, oral, narrow spectrum tetracycline-derived antibiotic with anti-inflammatory properties for the potential treatment of moderate to severe acne in the community setting.
"We are pleased that the FDA has accepted our application and look forward to advancing our conversations toward a potential U.S. approval of Seysara in the second half of 2018," said David Nicholson, Chief Research and Development Officer at Allergan. "This milestone reinforces our commitment to advancing research and bringing new therapies to patients, and our dermatology customers."

