The European Commission approves Skilarence® for moderate-to-severe chronic plaque Psoriasis
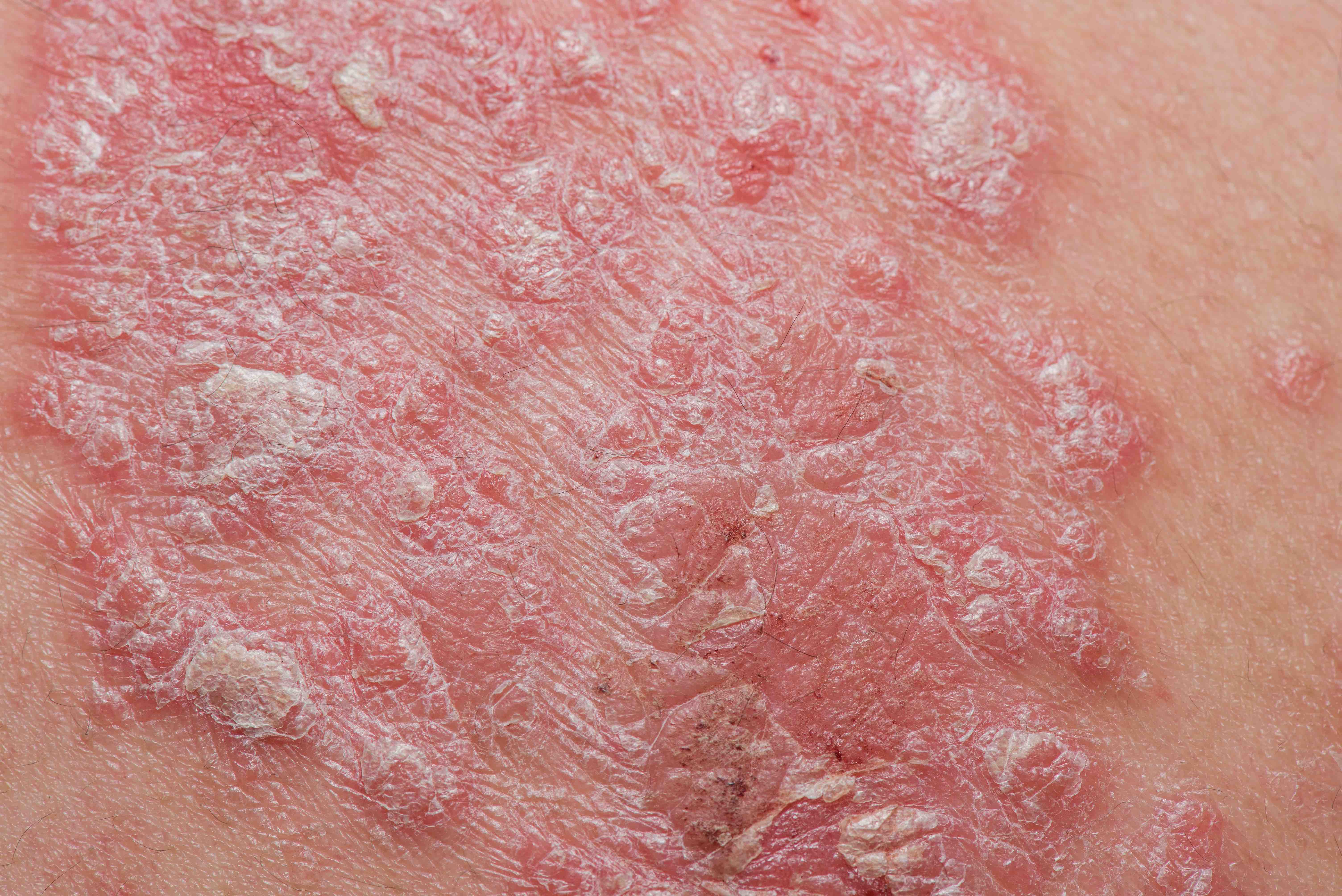 Almirall S.A. announced today that the European Commission (EC) has approved Skilarence®, a new oral formulation of dimethyl fumarate (DMF) developed by the pharmaceutical company, for the treatment for patients with moderate-to-severe chronic plaque Psoriasis. Skilarence® is to be indicated as a first-line induction and long-term maintenance treatment.
Almirall S.A. announced today that the European Commission (EC) has approved Skilarence®, a new oral formulation of dimethyl fumarate (DMF) developed by the pharmaceutical company, for the treatment for patients with moderate-to-severe chronic plaque Psoriasis. Skilarence® is to be indicated as a first-line induction and long-term maintenance treatment.
Almirall is due to start marketing Skilarence® in the third quarter of 2017 in all EU Member states, as well as in Iceland and Norway.
According to Eduardo Sanchiz, Almirall’s CEO, “the EC’s approval is very good news for healthcare professionals and for a large number of European patients, who will have access to a new therapeutic option for the systemic treatment of moderate-to-severe Psoriasis. Skilarence® is the result of Almirall’s commitment to innovation, and making it available to doctors and their patients with Psoriasis will constitute a very important step in reinforcing the company’s position as significant player in the field of Dermatology”.
Skilarence® is the first fumaric acid ester (FAE) for the treatment of Psoriasis approved by the EC. Its approval in Europe is based on the positive results from the randomised, double-blind, placebo-controlled Phase III trial (BRIDGE), evaluating the efficacy and safety of this new oral formulation of dimethyl fumarate compared to Fumaderm®, presented in September 2016 at the prestigious 25th EADV Congress in Vienna (Austria). Published in the British Journal of Dermatology, the BRIDGE trial showed the non-inferiority of DMF compared to Fumaderm® and a good efficacy and safety profile.1
To date, a fixed combination of fumaric acid esters was only available in Germany in a different composition as well as locally compounded formulations in some countries like the Netherlands, Austria and some Nordic countries. The EC’s decision will place this new oral formulation within the reach of all patients residing in all of the European countries.
Fumaric acid esters are a well-established oral systemic treatment for Psoriasis and are recommended in the European guidelines for both induction and long-term maintenance of the treatment of the disease. Long-term evidence coming from retrospective studies and registries highlights the clinical usefulness of the drug as well as its balanced safety profile.
References
- Mrowietz, U., Szepietowski, J.C., Loewe, R., van de Kerkhof, P., Lamarca, R., Ocker, W.G., Tebbs, V.M. and Pau-Charles, I. (2017), Efficacy and safety of LAS41008 (dimethyl fumarate) in adults with moderate-to-severe chronic plaque Psoriasis: a randomized, double-blind, Fumaderm®- and placebo-controlled trial (BRIDGE). Br J Dermatol, 176: 615–623. doi:10.1111/bjd.14947
- Nast A, Gisondi P, Ormerod AD et al. European S3-Guidelines on the systemic treatment of Psoriasis vulgaris - Update 2015 - Short version - EDF in cooperation with EADV and IPC. J Eur Acad Dermatol Venereol 2015; 29:2277-94.
- Mrowietz U, Altmeyer P, Bieber T et al. Treatment of Psoriasis with fumaric acid esters (Fumaderm). J Dtsch Dermatol Ges 2007; 5:716-7.
Reich K, Thaci D, Mrowietz U et al. Efficacy and safety of fumaric acid esters in the long-term treatment of Psoriasis, a retrospective study (FUTURE). J Dtsch Dermatol Ges 2009; 7:603-10. - Rostami-Yazdi M., Mrowietz U. Fumaric acid esters. Clin Dermatol 2008; 26:522-6.
