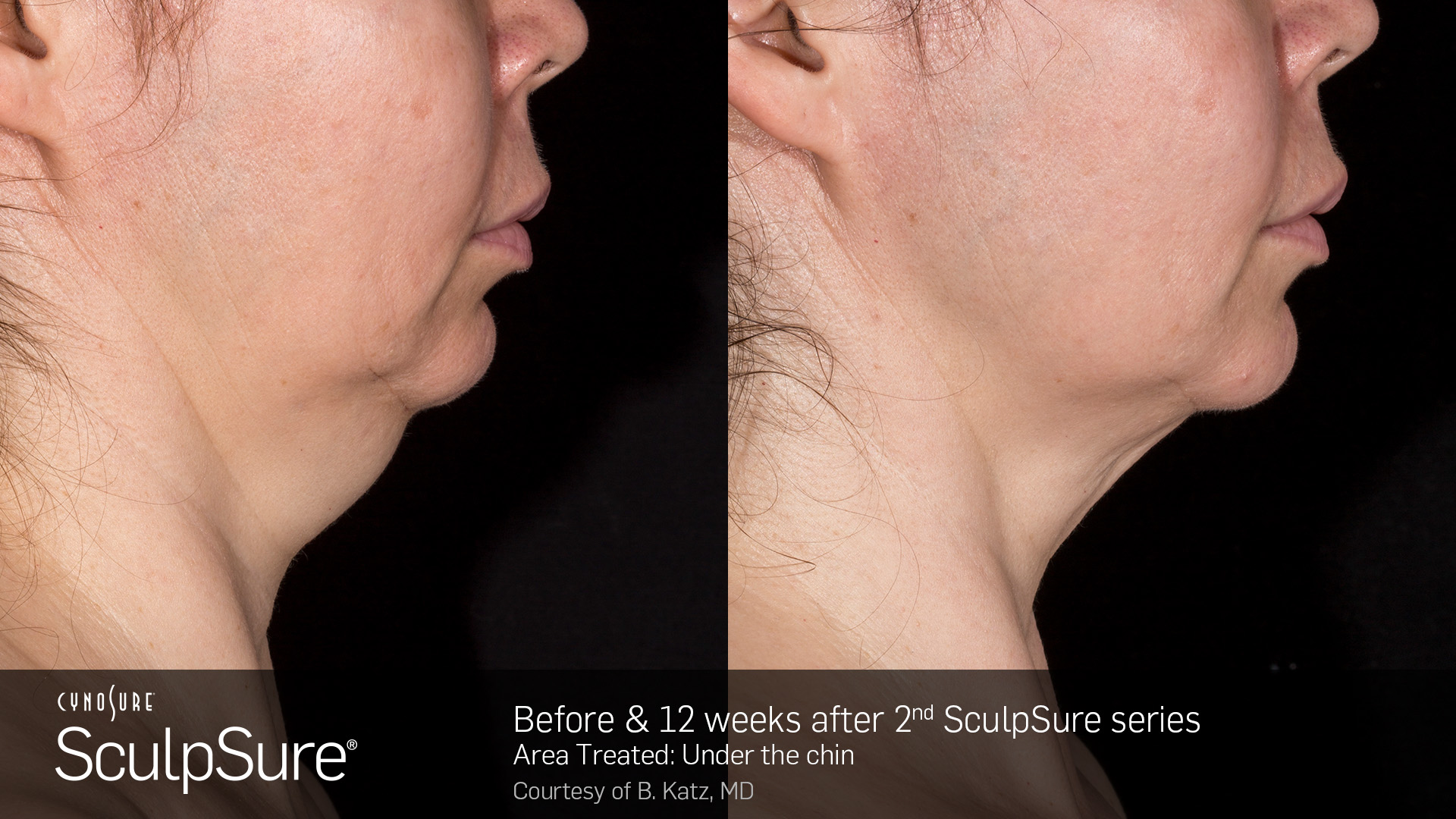
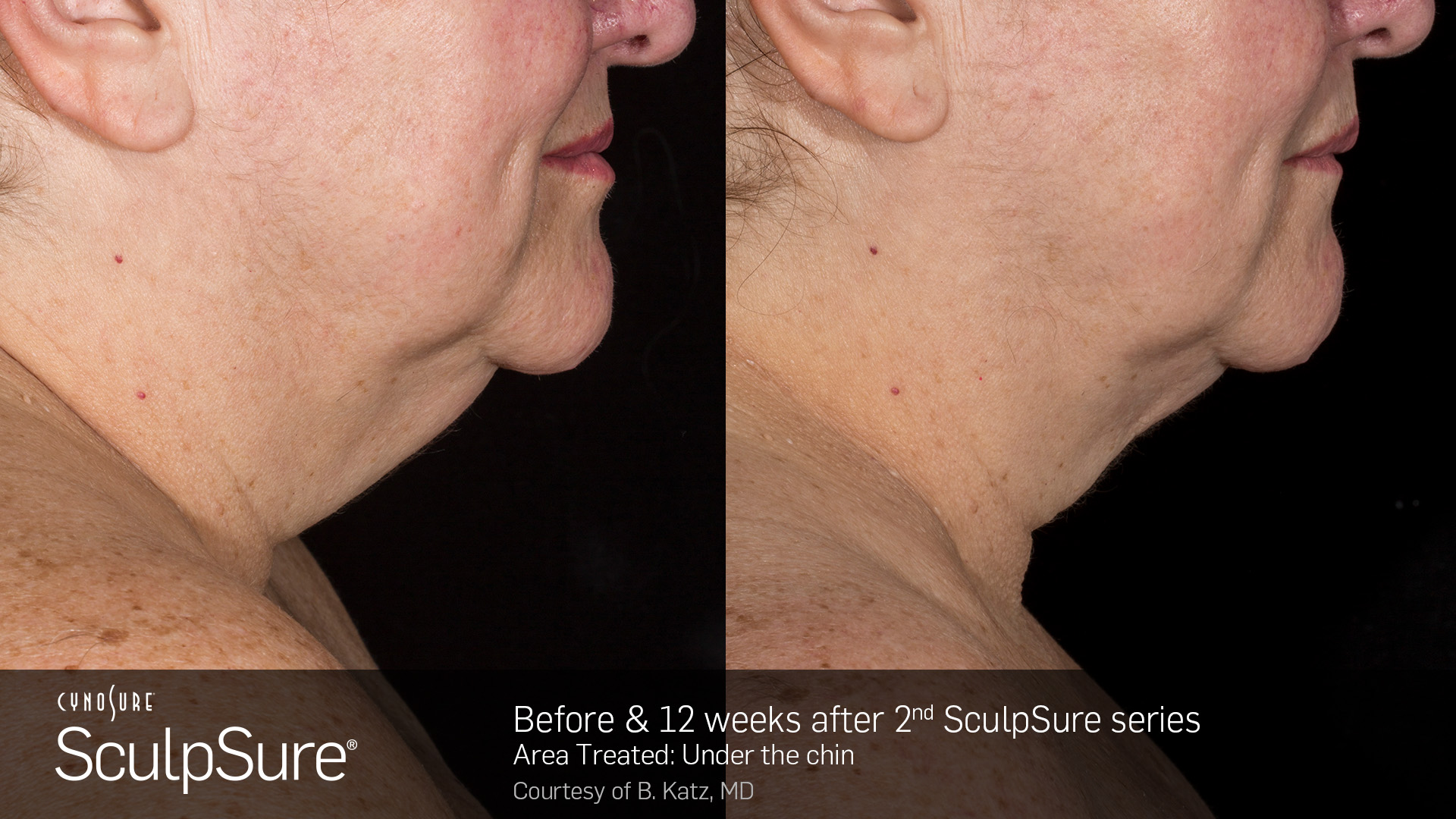
SculpSure receives CE mark for submental area
 Hologic has received an additional European Medical Device Directive (MDD) certification for SculpSure.
Hologic has received an additional European Medical Device Directive (MDD) certification for SculpSure.
The device is now certified to treat a double chin (also known as the submental area), marking the product’s sixth certified body treatment area. SculpSure is also certified to treat the abdomen, love handles (flanks), back, and inner and outer thighs. The certification allows Hologic to place the CE Mark on SculpSure double chin (submental) treatments for use in the European Union (EU) and its member states.
“Most patients in the 57-person submental clinical trial received two brief treatments six weeks apart,” said Dr. Lawrence Bass, board certified plastic surgeon and a principal investigator in the SculpSure clinical trials. “The short treatment time, 100% satisfaction rate, and dramatic contour reductions typically seen in the study patients give SculpSure the edge as the treatment of choice for the submental area.”
SculpSure is an advanced, noninvasive laser body contouring treatment that is fully customisable. The treatment raises the temperature of body fat to precisely disrupt and destroy fat cells under the skin. The fat cells are then naturally eliminated over time and do not return. Each treatment lasts approximately 25 minutes and requires no surgery or downtime. SculpSure is distributed by Cynosure.