Do fat-dissolving injections work?

Several adipocytolysis injectable products are available in the UK and are modifications of deoxycholate (DC): Aqualyx, Desoface and Desobody, Lipostabil and Lipodissolve.
Kybella is FDA-approved in the US and is known as Belkyra in Europe but is not yet available in the UK. Although greatly anticipated here, it appears patients elsewhere feel underwhelmed by the results. A recent search of Realself “worth it” ratings (accessed in May 2021) showed that 57% of 96 patients felt it was worthwhile, while 42% felt it was not.
Aqualyx has been in clinical use since 2009, mostly for reduction of localised adiposity, and I have used it in my clinic for three years. It is deemed a medical device in Europe and the manufacturer recommends its use alongside ultrasound for the microcavitation of adipose tissue. So, without ultrasound, Aqualyx on its own represents an off-label use of the medical device.
However, its solitary use has been extensively described in literature.2–4 Kybella and Belkyra, on the other hand, are recorded as drugs.4 The principal active ingredient of Aqualyx is deoxycholic acid. This is a secondary bile acid that is produced by the liver and used to break up fat for intestinal absorption. When injected into adipose tissue it disrupts the cell membranes and the fatty acid contents are released. The micro-gelatinous delivery system, which contains buffering agents and a low concentration of active detergent, appears to successfully reduce the inflammation caused by DC.5
Before I began working with Aqualyx, my insurer Medical Protection Society would not insure me for Lipodissolve use and there had been some controversy with the FDA about the safety claims of Lipostabil. However, both appear to have a good safety profile in the right hands and are widely used throughout Europe by the doctor group NETWORK-Lipolysis.
FAT REDUCTION AND SKIN STRENGTHENING
The marked immune response from Aqualyx treatment and resulting macrophage infiltrate appears to serve a two-fold purpose. First, the macrophages clear the cell membrane fragments and free lipids, and second, through fibroblast recruitment, neocollagenesis is stimulated.6,7 Once destroyed, the adipocytes cannot store or accumulate fat, resulting in fat reduction but also a bonus of tissue tightening.8,9
This explains why, in my experience, the tissue sagging that is commonly observed in a rapid weight-loss process is not seen in those successfully treated with Aqualyx.
CONTRAINDICATIONS
The contraindications to use are widely known and common to all the adipocytolysis injectable treatments. The side effect profile is what is expected; the transitory effects of the inflammatory sequalae in the skin are experienced by almost all and patients need to be counseled. Nodules are reported and are in the main reversible; they have been shown to be a consequence of inappropriate injection technique and their frequency was inversely proportional to the treating physician’s experience.3
Submental fat may re-accumulate following the procedure as the neighbouring unaffected fat cells can expand to fill the gap created by treatment. Like liposuction, there can be localised areas of depression. Skin numbness and neuralgia can also be a side effect. Lab and patient studies have shown direct injury of the marginal mandibular nerve (MMN) sheath with DC.10
It is presumed this occurs because the myelin sheath is predominantly lipid, so if the MMN is injured an asymmetric smile is seen (as it supplies the depressor labii inferioris muscle). In my research of papers and personal experience, this complication is transient and resolves spontaneously.
Dysphagia and beard area alopecia are also rare complications. There are rare reports of skin and muscle necrosis. Scientific studies show that the physiologic concentrations of albumin or protein-rich tissues will decrease the ability of DC to lyse cells but does not make these cells immune to apoptosis.11
In my three years of experience, I have only seen two of the rarer complications. One patient had hyperpigmentation and induration of the upper arm area that persisted for 12 months and the other was a temporary paralysis of the depressor labii inferior that lasted seven weeks. This complication occurred despite the recommended placement of the product 1.5cm below the inferior border of the
mandible from the gonion to the menton.12
However, it has been described in the literature that the nerve can be found up to 4cm below the inferior border of the mandible.8 In a Kybella trial, MMN paresis was identified as a possible complication but all were transitory.13
CASE STUDY
Mr GH is a 39-year-old patient who was concerned about submental fullness and lack of jawline definition. He attended the gym four times a week and his weight had stabilised at 91kg, making his BMI 27.5. He had a good diet and lifestyle. Over the years he has had toxin and both HA and bio-stimulating fillers (Ellansé). He grappled with insecurities about his appearance and these treatments gave him confidence to engage more freely in social interactions.
After a brief assessment, we discussed all available options including a targeted 5% weight loss through further dieting or oral or injectable medication (off license in his case), an attempt to improve the definition of the jawline through further treatment with Ellansé or with PDO/ PLLA/ PCL threads, a consideration of surgery with liposuction and/or neck lift; or some targeted adipocytolysis by the following methods: cavitation by high-intensity ultrasound, thermal damage through ablative radiofrequency, cryolipolysis, laser diode therapy or injectable adipocytolysis therapy.
He had been happy with filler in the past, but he saw that there was heaviness and hypertrophic adiposity in the submental area. These changes are frequently the result of ageing and weight gain but there is also a genetic component. This jowled appearance is further exacerbated by ptosis of the unsupported skin with the downward pull of the platysma muscle.1 He wanted to pursue adipocytolysis with injectable therapy. We could then visit defining the jawline with fillers or threads at a later date if needed.
Assesment
When assessing patients for adipocytolysis with injectable therapy, I manipulate the area to ascertain if it is adipose tissue and determine the thickness. If the area is thick there appears to be less risk of unsightly induration of the skin. I do a medical assessment and exclude other causes of fullness such as thyromegally and cervical lymphadenopathy.
I assess the skin for abnormal scarring tendencies and other contraindications and exclude these patients from treatment. I discuss how many treatments are most likely to produce good results. We then go through the consent and capacity process. I take a series of photos to include the profile view of the face with Frankfort plane parallel to the floor. I chart the patient’s weight.
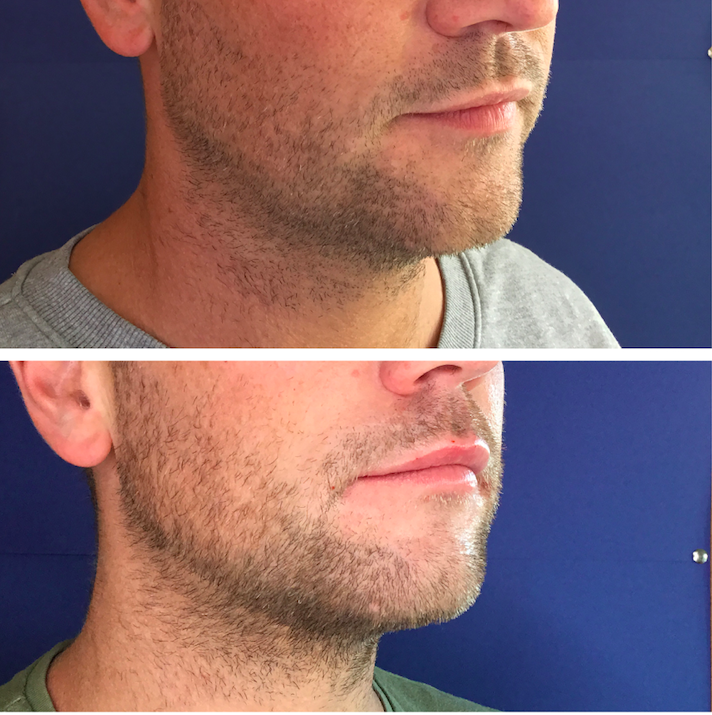
Top: before, Bottom: after two sessions of Aqualyx. Treatment by Dr Maeve Kenningham
Treatment and results
I use an ANTT and a sterile dressing pack. I add 0.2ml 1% lidocaine per vial as this aids my patient’s comfort, and use three X 3ml luer lok syringes with a 25G 70mm blunt cannula. The cannula is inserted in the pre-platysmal fat.
Threads of 0.3-0.5ml per pass of product are distributed as homogeneously as possible. I trained in the use of lipolysis sharp cannulas but found the bruising and haemosiderin staining an unnecessary draw back. I now use 27/25G 70mm blunt cannulas. This also helps in keeping my product in the low-resistance adipose plane.
The procedure itself takes less than 10 minutes to perform and the patient will be aware of heat and a burning sensation for the next 12 hours. The adipocytolysis occurs immediately on injection but the residual inflammation may take time to settle. The swelling tends to peak at day two to four – cold packs are helpful – and can last for six weeks, at which point I review to assess whether another treatment is needed.
Most patients require two to four treatments in the submental area. In this case, after two sessions of Aqualyx we did not have to proceed to further jawline sculpting as the patient was very happy with the results. His weight remained stable throughout.
RESULTS
The removal of fat cells will be permanent with adipocytolysis no matter what product is used. The resulting inflammation appears to have a skin strengthening fibrosis that further enhances the results.8,9 However, if weight is gained the neighbouring untreated adipocytes enlarge and fill the void left. I have a few patients who have had another submental treatment 18 months after their initial course and their results improve again without problems.
In my experience Aqualyx is a relatively safe and efficacious treatment for the reduction of submental fat. I look forward to comparing it to Belkyra when it appears on the UK market.
REFERENCES
1. The Anatomy of the Aging Face: Volume Loss and Changes in 3-Dimensional Topography. Sydney R. Coleman, MD, Rajiv Grover, BSc, MB BS, MD, FRCS (Plast) Author Notes. Aesthetic Surgery Journal, Volume 26, Issue 1_Supplement, January 2006, Pages S4–S9, https://doi.org/10.1016/j.asj.2005.09.012
2. Rauso R, Rusciani A, Curinga G. An adipocitolitic aqueous micro- gelatinous solution for buffalo hump deformity reduction. J Drugs Dermatol. 2014;13:1282–1284.
3. Pinto H, Hernandez C, Turra C, et al. Evaluation of a new adipocytolytic solution: adverse effects and their relation- ship with the number of vials injected. J Drugs Dermatol. 2014;13:1451–1455.
4. Rauso R, Salti G. A CE-marked drug used for localized adiposity reduction: a 4-year experience. Aesthet Surg J. 2015;35:850–857.
5: Comments on “Injection Lipolysis With Phosphatidylcholine and Deoxycholate” Giovanni Salti, MD, Raffaele Rauso, MD Aesthetic Surgery Journal, Volume 34, Issue 4, May 2014, Pages 639–640, https://doi.org/10.1177/1090820X14528506 Published: 01 May 2014
6. Duncan D, Rotunda AM. Injectable therapies for localized fat loss: state of the art, clinics in plastic surgery. Clin Plast Surg. 2011;38:489 501.[PubMed] [Google Scholar]
7. Saedi N, Rotunda AM, Dover JS. Injectable fatreducing therapies: fat reduction. In: Orringer JS, Murad A, Dover JS, editors. Body Shaping, Skin Fat and Cellulite: Procedures in Cosmetic Dermatology Series. Vol. 91 2014. [Google Scholar]
8. Plast Reconstr Surg Glob Open. 2016 Dec; 4(12 Suppl): e1155. Published online 2016 Dec 14. doi: 10.1097/GOX.0000000000001155 PMCID: PMC5172481 PMID: 28018773 Ava T. Shamban, MD
9. Pereira JX, Cavalcante Y, Wanzeler de Oliveira R. The role of inflammation in adipocytolytic nonsurgical esthetic procedures for body contouring. Clin Cosmet Investig Dermatol. 2017;10:57-66 https://doi.org/10.2147/CCID.S125580
10. Deoxycholic Acid and the Marginal Mandibular Nerve: A Cadaver Study June 2018. Aesthetic Plastic Surgery 42(Suppl 1) DOI:10.1007/s00266-018- 1164-4
11. Thuangtong R…Tissue selective effects of injected deoxycholate Dermatol Surg. 2010; 366: 899 - 908
12. Novel Expanded Safe Zone for Reduction of Submental Fullness with ATX-101 Injection. Shridharani, Sachin M. M.D.; Chandawarkar, Akash A. M.D. Author Information . Plastic and Reconstructive Surgery: December 2019 - Volume 144 - Issue 6 - p 995e-1001e doi: 10.1097/PRS.0000000000006299
13. Kythera Biopharmaceuticals, Inc. Dermatologic and ophthalmic drugs advisory committee briefing document: ATX-101 (deoxycholic acid) injection.

Dr Maeve Kenningham MBChB MRCGP DipDerm DRCOG DFFP is the director of Dr K’s Clinic in Wrexham, Wales, where she delivers relaxed, bespoke and patient-centred advanced medical aesthetic services. She also treats patients at NassifMD Medical Spa in Manchester once a fortnight. Dr Kenningham has been a GP for 20 years and has trained for the Royal College of General Practitioners. Follow her on Instagram: @dr_ks_clinic