FDA approves Botox Cosmetic for Forehead Lines
Published
22nd Oct 2017
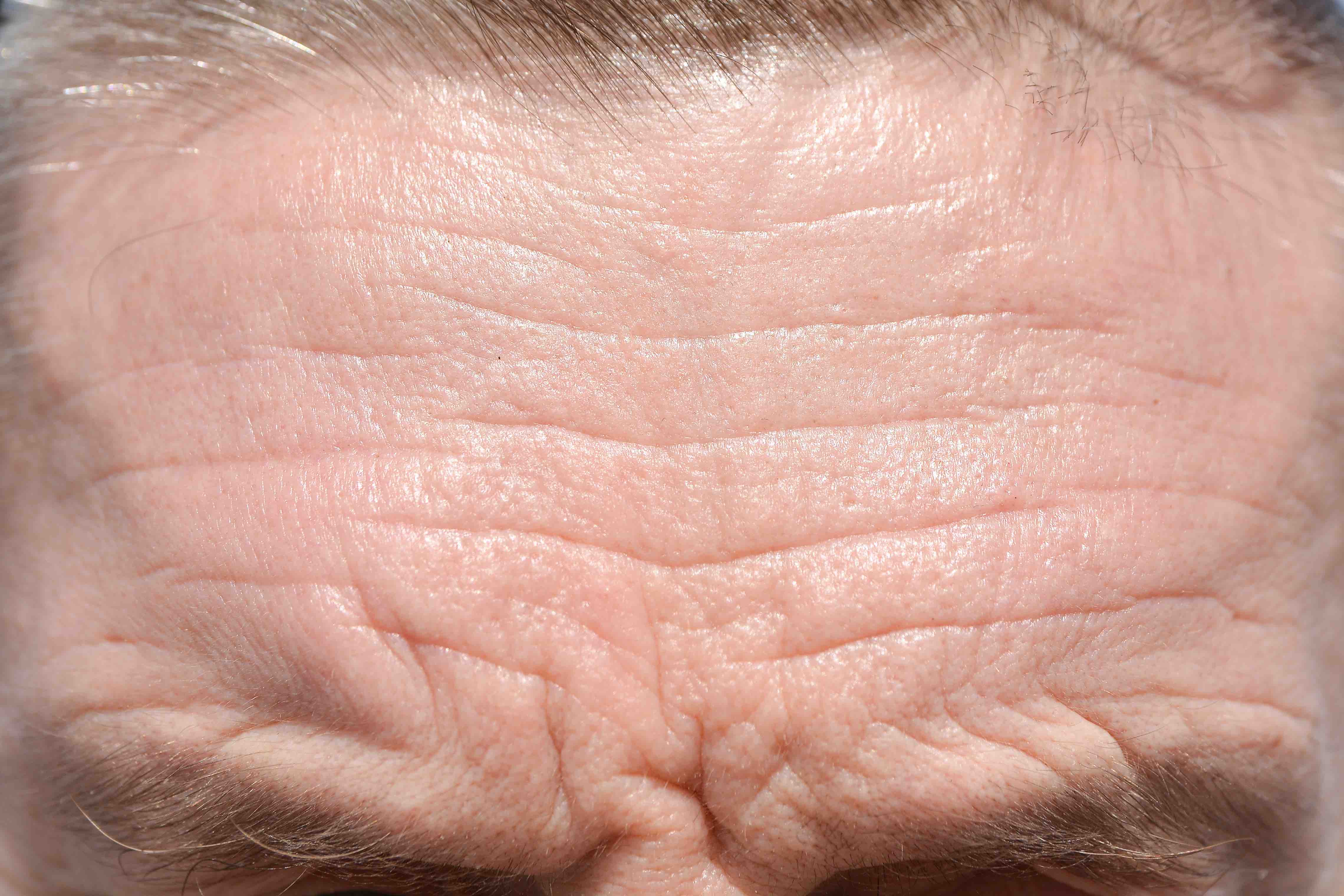 Allergan has announced the FDA approval of Botox Cosmetic for the temporary improvement in the appearance of moderate to severe forehead lines associated with frontalis muscle activity in adults. This approval makes the brand the first neurotoxin indicated for three facial treatment areas – forehead lines, crow’s feet lines and glabellar lines. Botox is also the only neurotoxin brand to receive approval of aesthetic indications beyond glabellar lines in the US.
Allergan has announced the FDA approval of Botox Cosmetic for the temporary improvement in the appearance of moderate to severe forehead lines associated with frontalis muscle activity in adults. This approval makes the brand the first neurotoxin indicated for three facial treatment areas – forehead lines, crow’s feet lines and glabellar lines. Botox is also the only neurotoxin brand to receive approval of aesthetic indications beyond glabellar lines in the US.

