Industry calls for statutory regulation following Times report on "black market botox"

The Times has published a damming report into "black market botox" highlighting the dangers of the unregulated aesthetics market. The story has sparked a fresh wave of calls for regulation in the sector from leading organisations such as The British College of Aesthetic Medicine (BCAM) and Joint Council for Cosmetic Practitioners (JCCP).
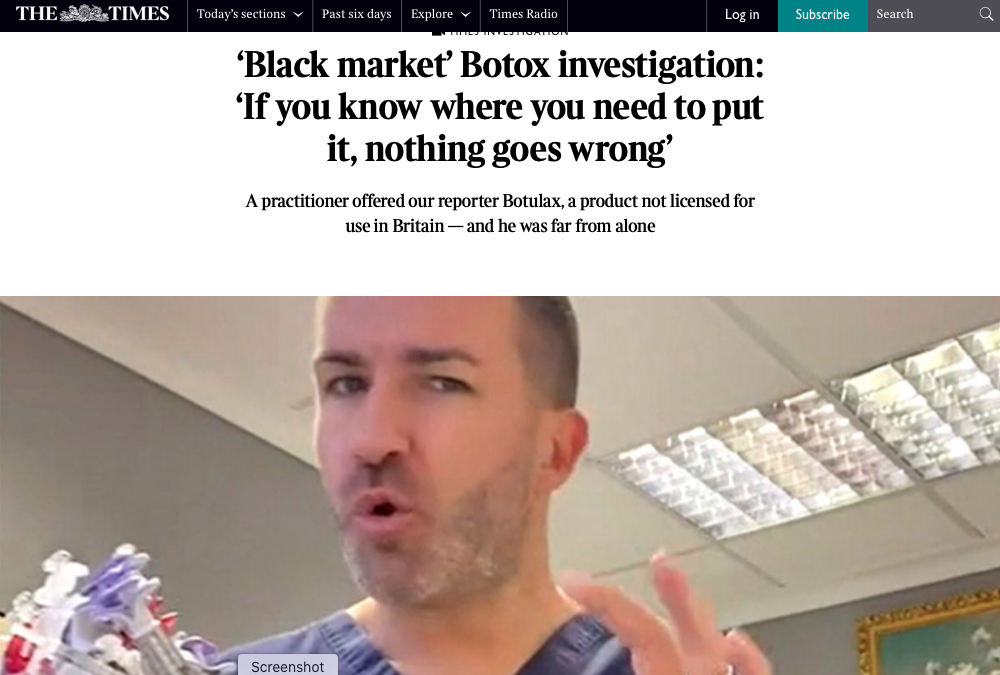
The article, which came out today (February 3), focussed on an undercover investigation of non-healthcare professionals injecting unlicensed botulinum toxin products bought on the black market.
It exposed a number of practitioners across the UK with no professional medical qualifications who advertise their services on social media, offering treatments using cut-price products that are not approved by the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) and injecting younger women ‘putting them at risk of being disfigured for life’.
 This afternoon both the JCCP and BCAM issued statements calling for statutory regulation of cosmetic treatments involving injectables stating these should only be administered by regulated healthcare professionals.
This afternoon both the JCCP and BCAM issued statements calling for statutory regulation of cosmetic treatments involving injectables stating these should only be administered by regulated healthcare professionals.
Professor David Sines CBE, the Chair and Registrar of the JCCP, said, “After discussion with practitioners, consumers, patients, stakeholders and politicians we are today calling for the Government to immediately introduce legislation to regulate the non-surgical aesthetic sector in the UK. We have concluded that in the interests of patient safety and public protection, high-risk and potentially harmful and invasive procedures, such as the injection of toxins, deep penetrative lasers, the administration of dermal fillers and the intravenous application of vitamins and ‘platelet rich’ plasma should only be administered by appropriately trained healthcare professionals.
“The JCCP receives an average of more than thirty complaints and ‘issues of concern’ each week regarding unsafe practice associated with treatments, medicines and the supply of aesthetic products and the training standards and qualifications that many practitioners present with.
“As a starting point, I would urge all Parliamentarians to support the amendment that the JCCP and others have tabled to the Health and Care Bill, currently progressing through Parliament, which would introduce a mandated licensing regime for the more invasive cosmetic treatments and make it an offence for someone to practise without a licence.”
In recent years the JCCP has witnessed a growing number of harmful complications arising from unlicensed drugs or poor treatment by inappropriately qualified and trained practitioners. JCCP has also become increasingly concerned about the use of unlicensed medicines and released a guidance statement on this last December. This guidance has been reviewed by UK professional regulators and notes that the use of unlicensed brands of botulinum toxin cannot currently be supported for cosmetic purposes. The JCCP also notes that the provision of unlicensed medicines, such as Botulax, or indeed any prescription medicine, without a valid prescription, is an offence.
BCAM President Dr Uliana Gout said: “The College strongly feels that this investigation has exposed a practise that we know is going unchecked and is putting members of the public at huge risk. BCAM is profoundly concerned about potentially life-changing injuries from using these illegal fake products and the misuse of genuine products by non-healthcare professionals.
“Immediate government action is needed in this virtually unregulated sector to protect the public from serious harm by limiting the use of these products exclusively to regulated healthcare professionals.
“Botulinum toxin is a prescription-only medicine that should be prescribed by a healthcare professional in a face-to-face consultation with the patient, yet it is being widely administered by practitioners with no medical training who are buying black-market supplies which are completely unregulated or receiving them from unscrupulous prescribers.”
In 2013 Sir Bruce Keogh published a report on cosmetic interventions that recommended a number of measures, including making fillers prescription-only devices, introducing a national register for practitioners and establishing accredited qualifications for non-surgical interventions. None of these measures have been adopted by the government.
The Health and Care Bill currently going through Parliament is expected to introduce new regulatory measures such as a licensing scheme for practitioners, but the extent is unclear.
BCAM represents around 400 doctors and dentists practising aesthetic medicine. The College believes all injectable treatments should be carried out only by qualified healthcare professionals who can prescribe and have the ability to deal with complications if they arise.
Dr Gout said: “Not only are members of the public at risk from these unregulated products being administered by people with no medical training, but there is also an issue if complications arise as the practitioner is unable to access the necessary prescription-only medicine to quickly put it right.
“BCAM’s Annual Clinical Review, an audit of members’ activity over the previous 12 months, last year highlighted a rise in the number of complications they had treated that originated from non-healthcare professionals. Aesthetic treatments such as fillers and botulinum toxin injections have become increasingly popular over the past couple of years. Hence, it’s crucial for the government to introduce urgent regulation to protect the public.”
The JCCP’s guidance on the use of unlicensed medicines can be found here.

