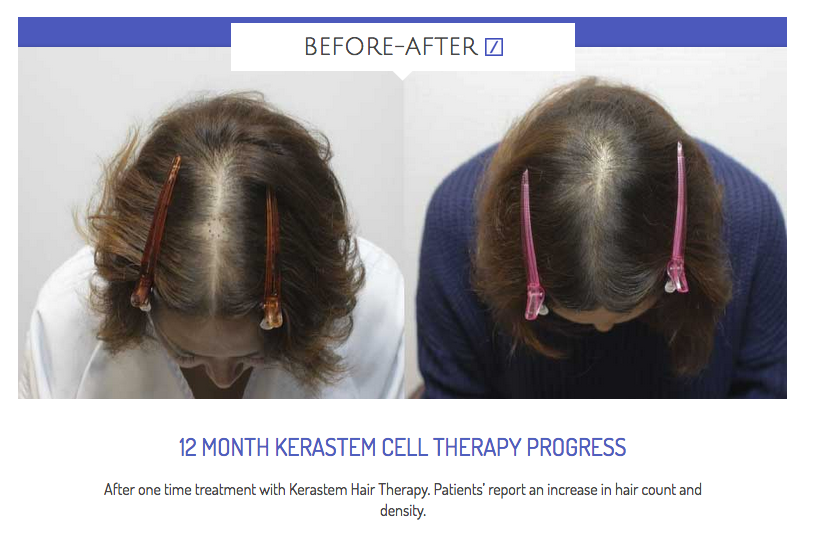
Kerastem reports successful phase II hair growth trial results
Published
08th Feb 2018
Kerastem has reported six-month top-line data from its 70 patient phase II clinical trial for STYLE.
The therapy utilises patented medical device technology to deliver a consistent cell therapy output, enabling appropriate dosing. It utilises adipose derived regenerative cells combined with purified fat delivered to the affected area of scalp.
The treatment group which recieved low dose Adipose Derived Regenerative Cells (ADRC’s) plus Puregraft fat achieved a statistically significant increase in mean terminal hair count (when compared to control) in males with early stage hair loss (Norwood Grades I-III). An average increase of 29 terminal hairs per cm2 of scalp was observed, corresponding to a 17% increase (p < 0.05) from baseline. All treatment arms of STYLE were safe with no serious adverse events reported.
Dr Eric Daniels, Kerastem’s chief medical officer, said, “STYLE indicates that dosing and tissue preparation are essential to achieving a successful outcome. “We were very pleased to see the positive results from the low dose arm and were surprised that the high dose ADRC’s plus Puregraft fat treatment group, along with the fat alone group, observed a reduction or no change respectively in terminal hair counts. Stated otherwise, dosing and tissue purity matter.”